Introduction
The quantification of blood flow in biological tissues is crucial for understanding physiological processes and diagnosing various medical conditions. One widely used optical technique for assessing tissue perfusion is Diffuse Correlation Spectroscopy (DCS), which measures the blood flow index (BFI) – an optically accessible parameter that serves as a proxy for tissue perfusion. BFI is defined as the product of the dynamic scattering probability (α) and an effective red blood cell (RBC) Brownian diffusion coefficient (DB).
Traditionally, DCS measurements have been performed in the near-infrared wavelength range, typically between 767-855 nm. However, the underlying assumption has been that BFI is wavelength-independent. This assumption has been rarely questioned, despite the potential for wavelength-dependent α to introduce inconsistencies in BFI measurements.
In this tutorial, we will explore a recent study that challenges the commonly held belief of wavelength-independent BFI. Using a dual-wavelength interferometric near-infrared spectroscopy (iNIRS) system, the researchers reveal that the dynamic scattering probability (α) and, consequently, the blood flow index (BFI) are indeed wavelength-dependent. We will also discuss the importance of incorporating higher-order mean squared displacement (MSD) cumulants in accurately modeling the field autocorrelation functions obtained from iNIRS measurements.
Dual-Wavelength iNIRS Setup
The key to this study's success was the development of a dual-wavelength iNIRS system, which allowed for simultaneous autocorrelation measurements at 773 nm and 855 nm (Figure 1). The system was designed to ensure that both wavelengths illuminated and collected light from the same spot on the tissue, minimizing any potential differences in the probed tissue regions.

The system employed two distributed feedback (DFB) lasers, one operating at 773 nm and the other at 855 nm. The lasers were sinusoidally modulated to achieve time-of-flight (TOF) resolution, with the modulation input voltages from a common function generator. This synchronized the wavelength tuning, ensuring that both wavelengths had matched TOF resolution.
The light from the two wavelengths was combined and delivered to the sample using a custom wavelength-division multiplexer (WDM). The remitted light was then separated by another WDM and recombined with the appropriate reference arm light before detection by dual-balanced detectors. This approach ensured that both wavelengths illuminated and collected light from the same spot on the tissue, a crucial requirement for meaningful comparisons of the autocorrelation functions.
Wavelength Dependence of Dynamic Scattering Probability (α)
The researchers investigated the wavelength dependence of α by applying two scaling methods to estimate the 773 nm autocorrelations from the 855 nm autocorrelations:
Constant α-scaling approach: This approach assumes that α is wavelength-independent, i.e., μ's,dyn = αμ's.
Constant μ's,dyn-scaling approach: This approach assumes that the dynamic scattering coefficient (μ's,dyn) is wavelength-independent, which implies that α is wavelength-dependent.

As shown in Figure 2, the constant α-scaling approach accurately predicted the 773 nm autocorrelations from the 855 nm autocorrelations in the Intralipid mixture, consistent with α = 1. However, in the in-vivo human forearm measurements, the constant μ's,dyn-scaling approach performed better, suggesting that α is indeed wavelength-dependent.
This wavelength dependence of α is further supported by the literature on RBC scattering, which indicates that the reduced scattering coefficient (μ's) of whole blood varies with wavelength, while the dynamic scattering (μ's,dyn) remains relatively constant (Figure 3). Consequently, the blood flow index (BFI = αDB) is also wavelength-dependent, as shown in Table 1.

Table 1. BFI of Intralipid and in-vivo forearm with their ratio across wavelengths

Importance of Higher-Order MSD Cumulants
The study also highlighted the importance of incorporating higher-order mean squared displacement (MSD) cumulants in accurately modeling the field autocorrelation functions obtained from iNIRS measurements. The researchers found that the commonly used single exponential model of Diffuse Wave Spectroscopy (DWS) [Eq. (2)] adequately described the autocorrelation functions for the Intralipid phantom (g_dyn ≈ 0.6), but failed to capture the slowly decaying tails of the in-vivo autocorrelation functions (g_dyn ≈ 0.98).



Eq. (1) :
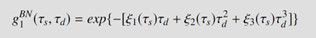

To better describe the in-vivo autocorrelation functions, the researchers employed a more comprehensive model [Eq. (1)] that incorporated higher-order MSD cumulants. This model was able to capture the slowly decaying tails of the autocorrelation functions, particularly at early and intermediate time-of-flights (Figures 18-20).
Eqs. (2), (3) and (4) :


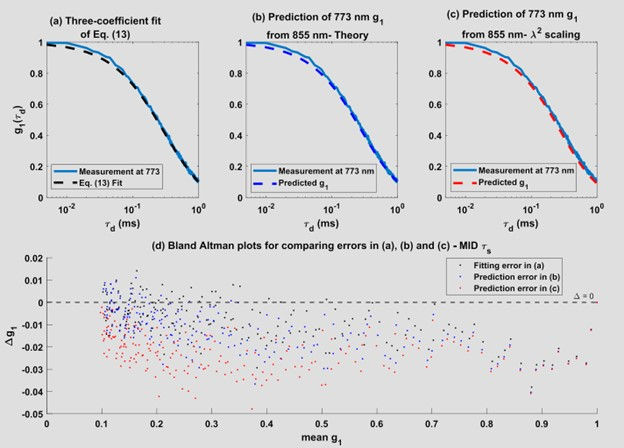

The researchers found that the higher-order MSD cumulant terms decreased with increasing wavelength, indicating that the tails of the autocorrelation functions are more pronounced at shorter wavelengths (773 nm) compared to longer wavelengths (855 nm). This observation aligns with the theoretical predictions and underscores the importance of considering higher-order MSD cumulants in accurate modeling of autocorrelation functions, particularly in applications such as laser speckle and DCS.
Conclusion
This tutorial has highlighted the key findings from a recent study that challenged the commonly held assumption of wavelength-independent blood flow index (BFI) in diffuse correlation spectroscopy. Using a dual-wavelength iNIRS system, the researchers demonstrated that the dynamic scattering probability (α) and, consequently, the BFI are indeed wavelength-dependent. This finding has important implications for the interpretation and comparison of BFI measurements obtained using different wavelengths.
Furthermore, the study emphasized the significance of incorporating higher-order mean squared displacement (MSD) cumulants in accurately modeling the field autocorrelation functions, particularly in biological tissues with high dynamic scattering anisotropy. This insight is crucial for improving the interpretation of laser speckle, DCS, and related modalities that rely on autocorrelation analysis.
The dual-wavelength iNIRS approach, along with the theoretical framework developed in this study, provides a robust methodology for investigating the wavelength dependence of optical parameters in biological tissues. These findings underscore the importance of careful consideration of wavelength-dependent effects in the interpretation of diffuse optics measurements, ultimately enhancing our understanding of tissue perfusion and other physiological processes.
Reference
[2] D. Mazumder, O. Kholiqov, and V. J. Srinivasan, "Interferometric near-infrared spectroscopy (iNIRS) reveals that blood flow index depends on wavelength," Biomedical Optics Express, vol. 15, no. 4, pp. 2152-2173, Apr. 2024. https://doi.org/10.1364/BOE.507373

Comments